Now you have the choice to do more than ‘watch and wait’ for your patients with la/mUC WHO ARE PROGRESSION-FREE FOLLOWING PBCT1–5
BAVENCIO + BSC – the JAVELIN Bladder 100 trial
JAVELIN Bladder 100 was a Phase III, randomised, open‑label trial that investigated BAVENCIO + BSC versus BSC alone as first‑line maintenance therapy for adult patients with unresectable la/mUC who did not experience disease progression with first‑line PBCT. The primary endpoint of JAVELIN Bladder 100 was OS; secondary endpoints included PFS, ORR, TTR, DOR, disease control and safety.2,4
Treatment with BAVENCIO + BSC (n=350) significantly improved mOS by 8.8 months versus BSC alone (n=350) based on the ≥2-year follow-up data (23.8 months versus 15.0 months; stratified HR: 0.76 [95% CI: 0.63–0.92]; p=0.0036).*2
Treatment with BAVENCIO + BSC (n=350) significantly improved mOS by 8.8 months versus BSC alone (n=350) based on the ≥2-year follow-up data (23.8 months versus 15.0 months; stratified HR: 0.76 [95% CI: 0.63–0.92]; p=0.0036).*2
BAVENCIO + BSC improved mOS from the start of first-line chemotherapy versus BSC alone (post hoc analysis)1
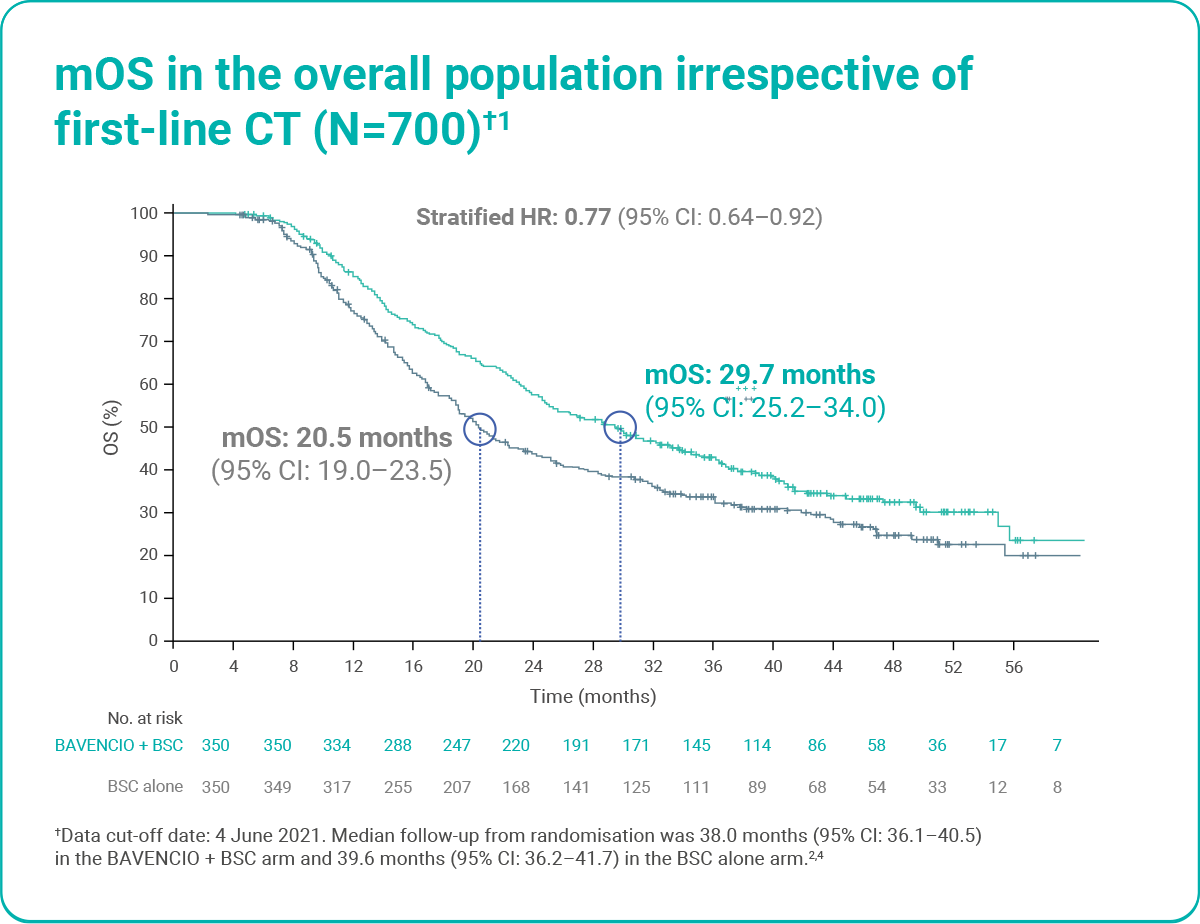
Figure adapted from Sridhar S et al. 2023.1

OS was longer with BAVENCIO + BSC versus BSC alone, irrespective of first-line CT regimen (post hoc analysis)1
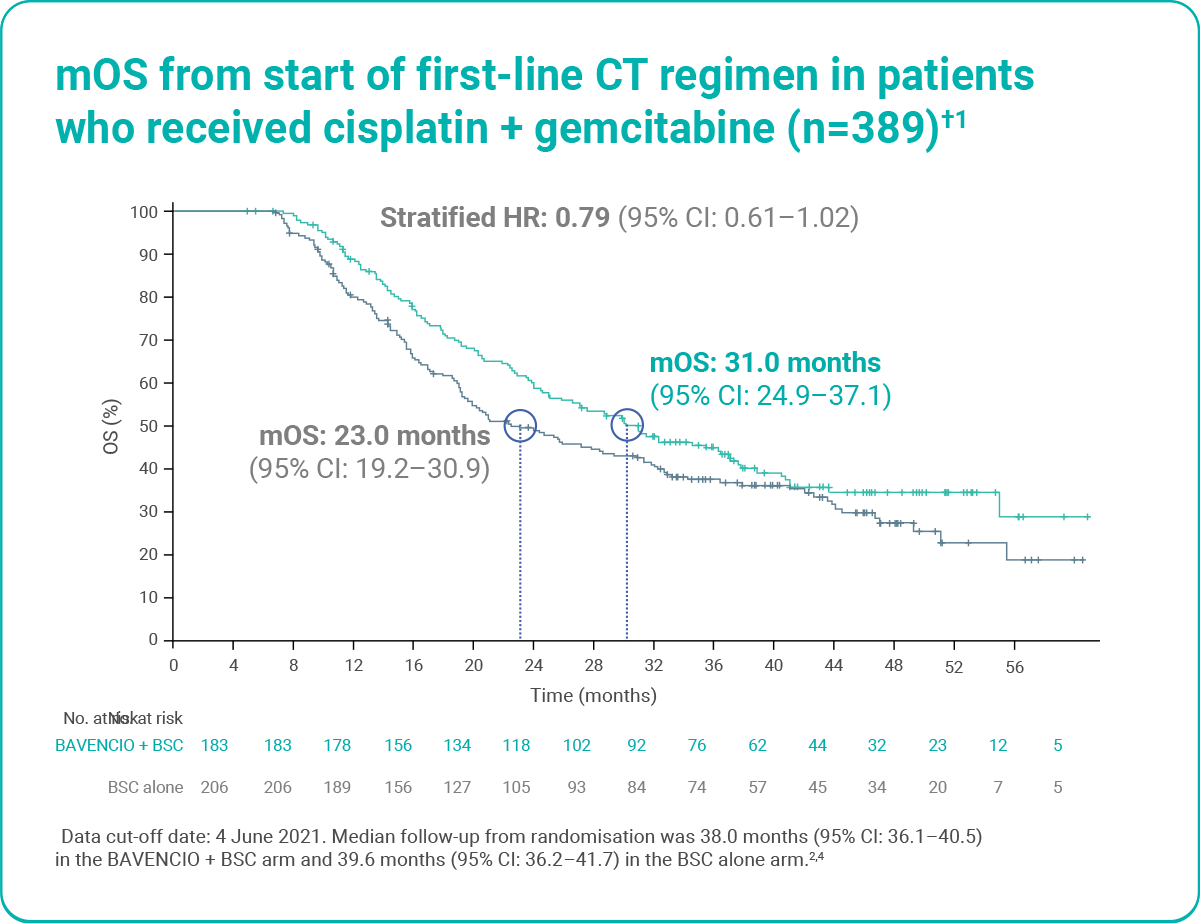
Figure adapted from Sridhar S et al. 2023.1

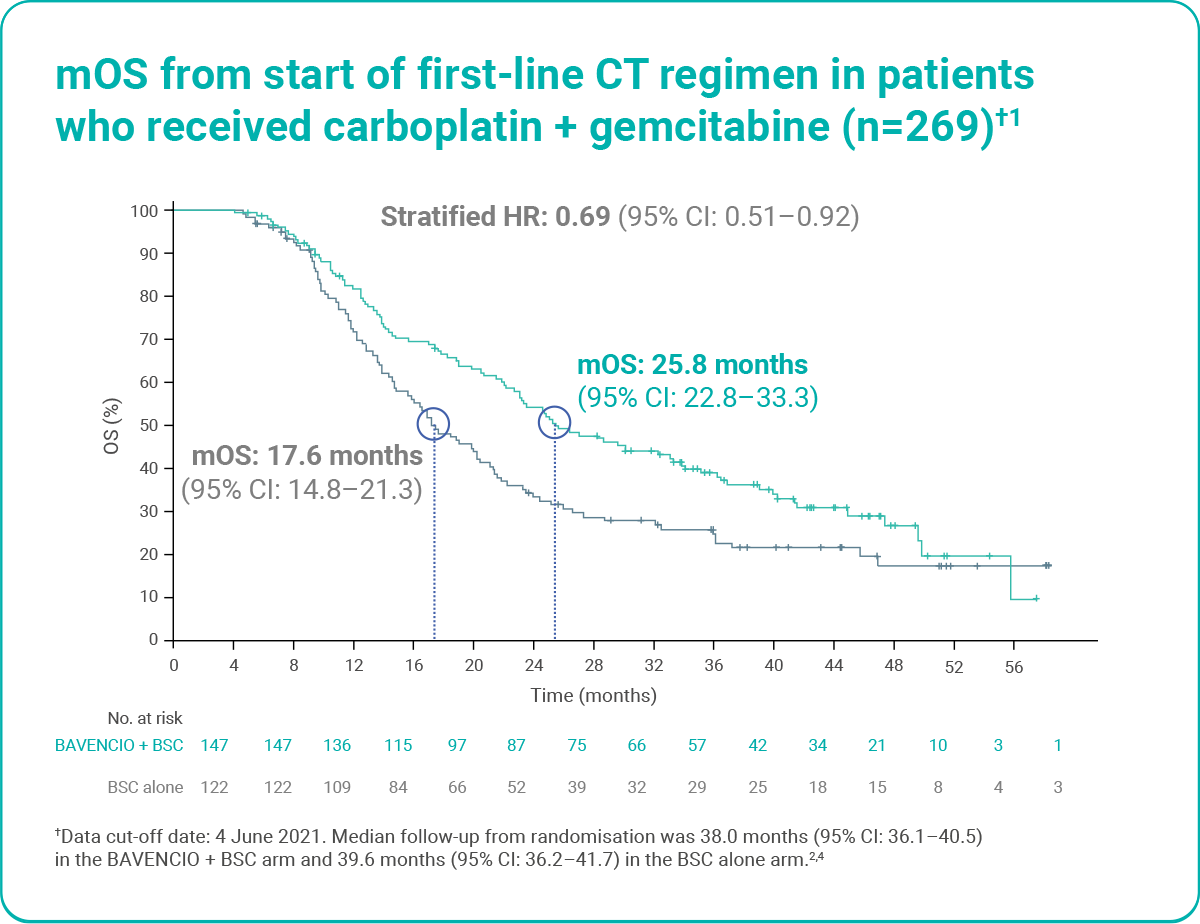
Figure adapted from Sridhar S et al. 2023.1

BAVENCIO first-line maintenance therapy is a recommended immunotherapy option for adult patients with la/mUC who have not progressed after first-line PBCT, based on the highest-level (category 1) evidence from the international organisations NCCN, ESMO and EAU5–9
BAVENCIO + BSC demonstrated no detrimental effect on clinically relevant PROs versus BSC alone10

Figure adapted from Grivas P et al. 2023.10
No clinically important differences were observed in QoL in patients treated with BAVENCIO + BSC versus BSC alone (FBlSI-18 total score 52.51 [95% CI: 51.54–53.48] vs 52.41 [95% CI: 51.25–23.59])10
More patients treated with BSC alone versus BAVENCIO + BSC received subsequent treatment (52.9% [n=185/350] vs 72.0% [n=252/350])†2
Overall, 11.4% of patients who received BAVENCIO + BSC (n=40/350) received subsequent treatment with PD-1/PD-L1 inhibitors versus 53.1% of patients who received BSC alone (n=186/350)†2
Overall, 11.4% of patients who received BAVENCIO + BSC (n=40/350) received subsequent treatment with PD-1/PD-L1 inhibitors versus 53.1% of patients who received BSC alone (n=186/350)†2
Bavencio maintenance treatment was generally well tolerated
Long-term safety data for first-line maintenance treatment of combined CR, PR and SD subgroups in the BAVENCIO + BSC arm†11
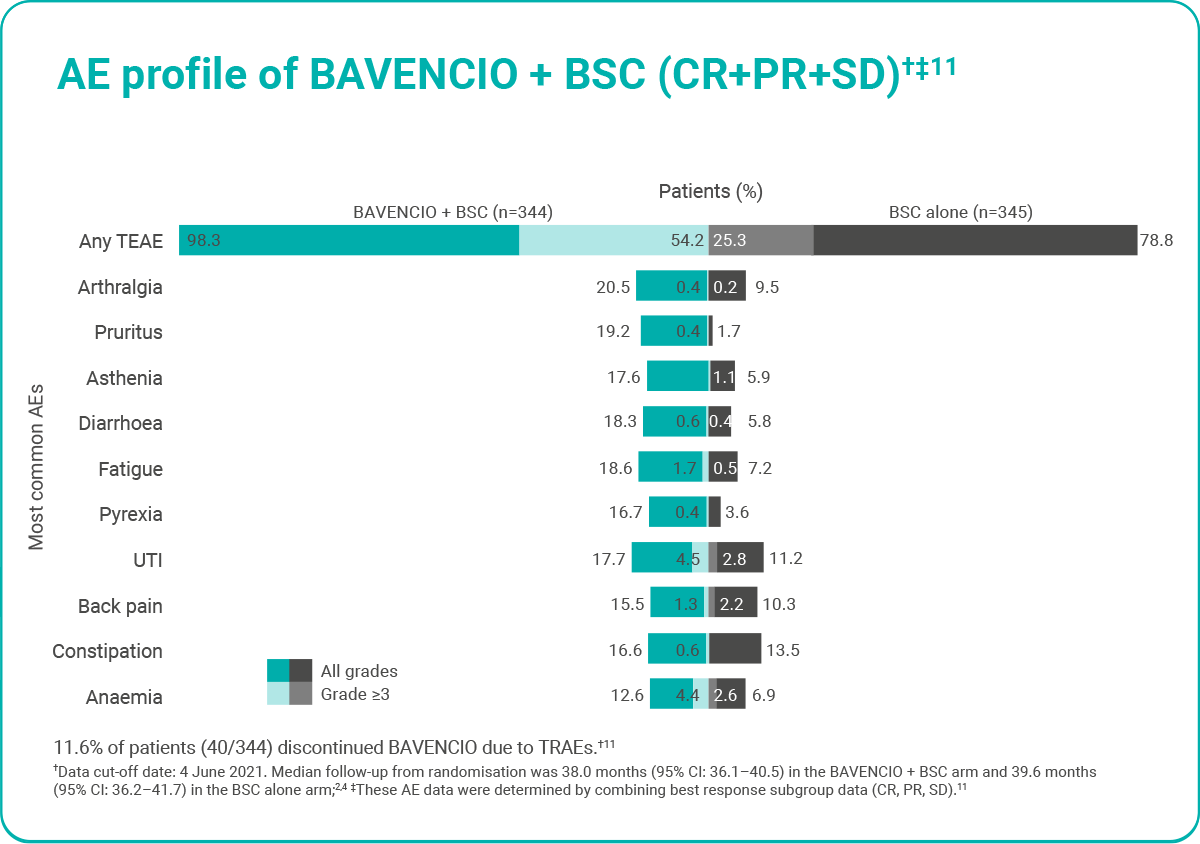
Figure adapted from Valderrama BP et al. 2022.11
In JAVELIN Bladder 100, most irAEs associated with BAVENCIO were reversible and managed with temporary discontinuation of BAVENCIO, administration of corticosteroids and/or supportive care3
In the BAVENCIO + BSC arm, 12.3% of patients (n=43) were still receiving study treatment at data cut-off (4 June 2021)†2



